Equation Of Baking Soda Formula . Here is a look at the reaction between vinegar and baking soda and the equation for the reaction. The compound is a salt that dissociates into. the molecular formula of sodium bicarbonate is nahco 3. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. the reaction between baking soda (sodium bicarbonate) and vinegar (dilute acetic acid) generates carbon dioxide gas, which is used in chemical volcanoes and other projects. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. sodium bicarbonate (nahco3), white crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking.
from www.alamy.com
one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. sodium bicarbonate (nahco3), white crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking. the molecular formula of sodium bicarbonate is nahco 3. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. Here is a look at the reaction between vinegar and baking soda and the equation for the reaction. sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. The compound is a salt that dissociates into. the reaction between baking soda (sodium bicarbonate) and vinegar (dilute acetic acid) generates carbon dioxide gas, which is used in chemical volcanoes and other projects.
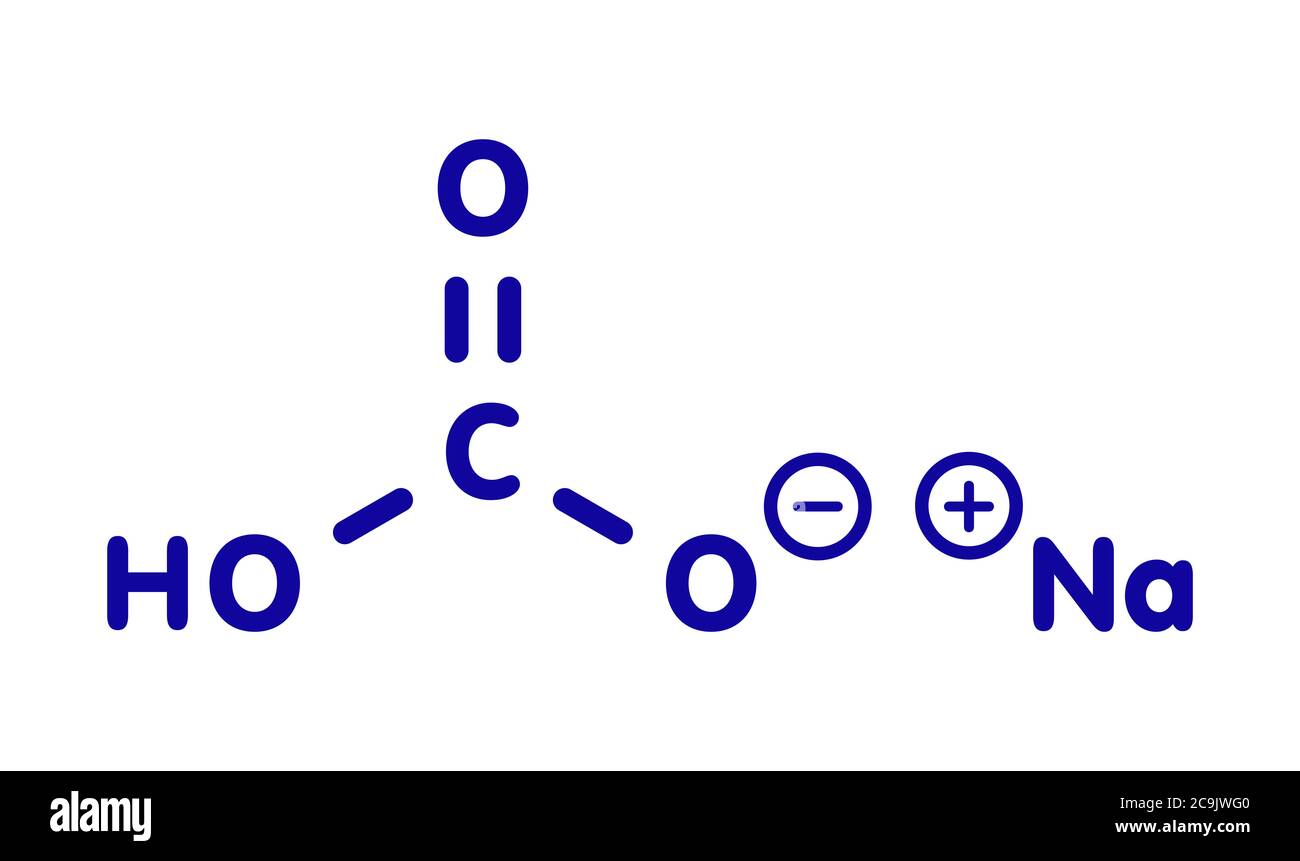
Sodium bicarbonate (baking soda), chemical structure. Blue skeletal
Equation Of Baking Soda Formula The compound is a salt that dissociates into. the reaction between baking soda (sodium bicarbonate) and vinegar (dilute acetic acid) generates carbon dioxide gas, which is used in chemical volcanoes and other projects. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. The compound is a salt that dissociates into. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. sodium bicarbonate (nahco3), white crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking. the molecular formula of sodium bicarbonate is nahco 3. Here is a look at the reaction between vinegar and baking soda and the equation for the reaction.
From www.youtube.com
Write a chemical equation to deceribe how baking soda is produced on a Equation Of Baking Soda Formula sodium bicarbonate (nahco3), white crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking. The compound is a salt that dissociates into. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. the molecular formula of sodium. Equation Of Baking Soda Formula.
From www.congress-intercultural.eu
Chemical Formula Of Baking Soda Outlet site www.congress Equation Of Baking Soda Formula sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. the molecular formula of sodium bicarbonate is nahco 3. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. one mole of sodium bicarbonate (baking soda) reacts. Equation Of Baking Soda Formula.
From ar.inspiredpencil.com
Chemical Formula For Baking Soda Equation Of Baking Soda Formula this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. The compound is a salt that dissociates into. sodium bicarbonate (nahco3), white crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking. sodium bicarbonate reacts with acids. Equation Of Baking Soda Formula.
From www.slideserve.com
PPT Baking Soda/Vinegar Stoichiometry Lab PowerPoint Presentation Equation Of Baking Soda Formula the reaction between baking soda (sodium bicarbonate) and vinegar (dilute acetic acid) generates carbon dioxide gas, which is used in chemical volcanoes and other projects. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. sodium bicarbonate (nahco3), white crystalline or powdery solid that is a source. Equation Of Baking Soda Formula.
From stock.adobe.com
Sodium bicarbonate (baking soda), chemical structure. Skeletal formula Equation Of Baking Soda Formula this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. the reaction between baking soda (sodium bicarbonate) and vinegar (dilute acetic acid) generates carbon dioxide gas, which is. Equation Of Baking Soda Formula.
From www.dreamstime.com
Sodium Bicarbonate Baking Soda, Chemical Structure. Skeletal Formula Equation Of Baking Soda Formula this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. Here is a look at the reaction between vinegar and baking soda and the equation for the reaction. sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. The. Equation Of Baking Soda Formula.
From exobgmxdk.blob.core.windows.net
Baking Soda And Water Reaction Equation at James Westberg blog Equation Of Baking Soda Formula one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. Here is a look at the reaction between vinegar and baking soda and the equation for the reaction. sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning.. Equation Of Baking Soda Formula.
From fr.dreamstime.com
Sodium Bicarbonate Molecule, Known As Baking Soda. Structural Chemical Equation Of Baking Soda Formula sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. Here is a look at the reaction between vinegar and baking soda and the equation for the reaction. . Equation Of Baking Soda Formula.
From exobgmxdk.blob.core.windows.net
Baking Soda And Water Reaction Equation at James Westberg blog Equation Of Baking Soda Formula the molecular formula of sodium bicarbonate is nahco 3. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. The compound is a salt that dissociates into.. Equation Of Baking Soda Formula.
From www.alamy.com
Sodium bicarbonate (baking soda), chemical structure. Skeletal formula Equation Of Baking Soda Formula the molecular formula of sodium bicarbonate is nahco 3. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. the reaction between baking soda (sodium bicarbonate) and vinegar (dilute acetic acid) generates carbon dioxide gas, which is used in chemical volcanoes and other projects. this. Equation Of Baking Soda Formula.
From asideload7.gitlab.io
Amazing Balanced Chemical Equation Of Vinegar And Baking Soda Equation Of Baking Soda Formula sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. Here is a look at the reaction between vinegar and baking soda and the equation for the reaction.. Equation Of Baking Soda Formula.
From www.pinterest.com
Know the Equation for the Baking Soda and Vinegar Reaction Baking Equation Of Baking Soda Formula one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. sodium bicarbonate (nahco3), white crystalline or powdery solid that is a source of carbon dioxide and. Equation Of Baking Soda Formula.
From exotjzliw.blob.core.windows.net
Vinegar + Baking Soda Chemical Equation at Allen Oneal blog Equation Of Baking Soda Formula the molecular formula of sodium bicarbonate is nahco 3. sodium bicarbonate (nahco3), white crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in baking. The compound is a salt that dissociates into. sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in. Equation Of Baking Soda Formula.
From www.congress-intercultural.eu
Sodium Bicarbonate Molecule, Known As Baking Structural, 42 OFF Equation Of Baking Soda Formula the reaction between baking soda (sodium bicarbonate) and vinegar (dilute acetic acid) generates carbon dioxide gas, which is used in chemical volcanoes and other projects. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. the molecular formula of sodium bicarbonate is nahco 3. this. Equation Of Baking Soda Formula.
From www.youtube.com
Baking Soda and Vinegar Equation and Reaction YouTube Equation Of Baking Soda Formula Here is a look at the reaction between vinegar and baking soda and the equation for the reaction. sodium bicarbonate reacts with acids to produce salt, water, and carbon dioxide (co₂) gas, useful in baking and cleaning. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. . Equation Of Baking Soda Formula.
From shotprofessional22.gitlab.io
Supreme Baking Soda And Vinegar Word Equation C Formula Physics Equation Of Baking Soda Formula the molecular formula of sodium bicarbonate is nahco 3. this is the balanced chemical equation for the decomposition of sodium bicarbonate, or baking soda, by heat or in water. the reaction between baking soda (sodium bicarbonate) and vinegar (dilute acetic acid) generates carbon dioxide gas, which is used in chemical volcanoes and other projects. Here is a. Equation Of Baking Soda Formula.
From learningschoolfridell27.z4.web.core.windows.net
Peroxide And Baking Soda Reaction Equation Of Baking Soda Formula one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of. The compound is a salt that dissociates into. the molecular formula of sodium bicarbonate is nahco 3. Here is a look at the reaction between vinegar and baking soda and the equation for the reaction. sodium. Equation Of Baking Soda Formula.
From www.numerade.com
SOLVED Goal Write the thermochemical equation for the standard heat Equation Of Baking Soda Formula the reaction between baking soda (sodium bicarbonate) and vinegar (dilute acetic acid) generates carbon dioxide gas, which is used in chemical volcanoes and other projects. the molecular formula of sodium bicarbonate is nahco 3. sodium bicarbonate (nahco3), white crystalline or powdery solid that is a source of carbon dioxide and so is used as an ingredient in. Equation Of Baking Soda Formula.